Membranes and Functional Films
CROSS-FLOW FILTRATION
Cross-flow filtration is a procedural mode of operation for the separation of substances using membranes. A mixture of substances (feed, liquid or gaseous) is fed into a membrane module, with the mixture flowing over the membrane. By means of an applied driving force (pressure or concentration gradient), one component is extracted from the mixture through the membrane (permeate). The remaining mixture (retentate) is usually recycled and added to the feed in order to achieve a circulation process.
DEAD-END FILTRATION
In dead-end filtration, a mixture of multiple components is applied to a (porous) membrane until the component that is to be removed is eliminated from the mixture or until the membrane is no longer able to separate. In a continuous process, however, feed keeps being added. This process is usually employed in macro-, micro- and ultrafiltration, which are mostly used to separate solids from liquids. To prevent the membrane from clogging or to return an already clogged membrane to a functional state, it is backwashed. This means that the membrane is flushed from its permeate side to clean the solids off the pores.
DRIVING FORCE
The driving force is caused by a gradient of the chemical potential along the membrane. In practice, a pressure or concentration gradient is used for this purpose. Without the driving force, only the Brownian molecular motion characterizes the movement of the molecules or atoms.
FILTRATION
In membrane technology, various methods of filtration are employed. Commercially used filtration methods, their respective applications and pore sizes are summarized in the following table:
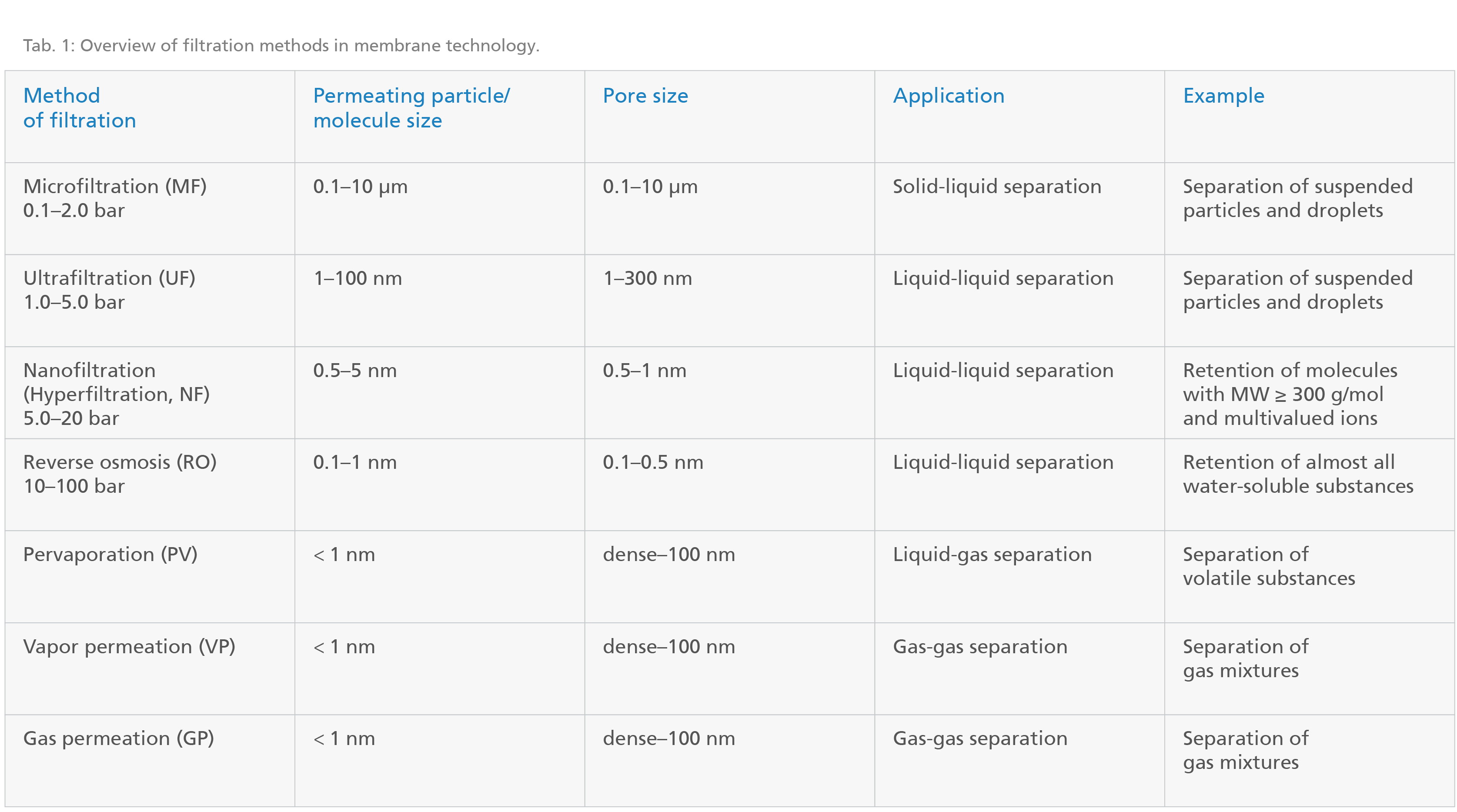
Porous membranes are used for micro-, ultra- and nanofiltration. Generally, a membrane is considered porous if its permanent pores have a diameter of 0.5 to 1 nm. For pervaporation, vapor permeation and gas permeation, on the other hand, dense membranes are used (pores smaller than 0.5 to 1 nm). Vapor and gas permeation differ in that gas permeation is utilized to separate permanent gases whereas vapor permeation is employed to separate gases which are liquid at room temperature and pressure.
FLUX
The substance flow related to the membrane area is called flux. The flux is often indicated with Ji, where i represents the respective component. It is the quotient of the mass m and the product of the area A of the membrane and time t. The determining factor is thus how much mass flows through the membrane per area and unit of time.
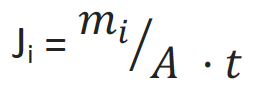
MEMBRANE
A membrane is a semi-permeable (semi, Lat. for: half, partial and permeare, Lat. for: pass (through)) barrier. This means that the membrane retains certain components of a multi-component mixture while others can pass. Membranes can be roughly divided into biological and synthetic membranes. For practical applications synthetic membranes are used, which in turn can be divided into inorganic (e.g. metal or ceramic membranes) and organic (polymer membranes) membranes. It is possible to produce composite membranes that consist of multiple materials as well (e.g. multi-layer structures of membranes or mixed-matrix membranes). In practice, it is difficult to create a membrane with chemical, thermal and mechanical stability that is also durable and cost-efficient and has a good separation efficiency. A good separation efficiency is achieved through high permeability at high selectivity.
PERMEABILITY
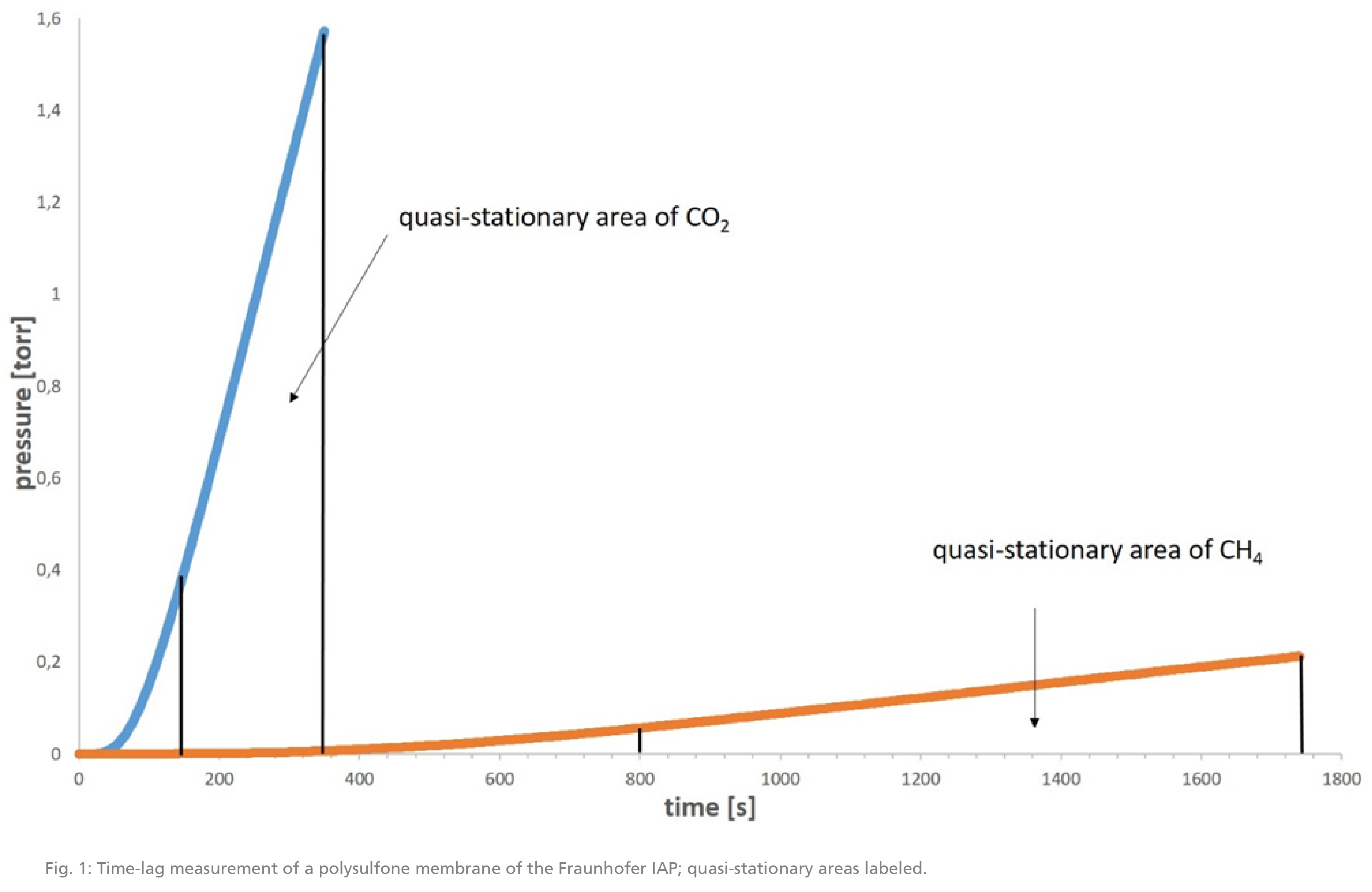
To determine the separation efficiency of gas separation membranes, permeability measurements are usually performed, e.g. the time-lag method. For this purpose, a membrane located in a test cell is first evacuated. Then, a gas is applied to the membrane and the increase in pressure on the permeate side is documented. Based on the amount of permeated gas, the time required for permeation, the membrane area and the pressure on the feed side, the permeability can be calculated. With the time-lag method in particular, one must wait until the quasi-stationary state is reached (s. Fig. 1).
The ideal separation factor of a membrane can be determined by measuring different gases.
PERMEATION
Permeation is the movement of one substance through another. In membrane technology, this means the movement of a liquid or a gas through a membrane. Permeation can be divided into three steps:
- the sorption of a gas or a liquid from the surface of a membrane into the membrane,
- the diffusion of the dissolved gas or liquid, respectively, along the driving force across the membrane and
- the desorption of the dissolved molecules or atoms from the surface of the membrane’s permeate side.
The driving force mentioned in point 2 is generated artificially; usually in the form of pressure differences between the two sides of the membrane (feed and permeate). In the case of porous membranes, both fluids and solids permeate through the pores of the membrane.
PERVAPORATION
Pervaporation (often abbreviated as PV) is the separation of mixtures of liquids. To achieve this, a dense membrane is used, in which the liquid must dissolve before it can permeate through the membrane. On the permeate side, liquids are removed in the form of vapor via a vacuum or a sweep gas. The solubility of the membrane’s components is another important factor that influences the separation efficiency during pervaporation. The component with the higher solubility and/or the smaller molecular size is more likely to permeate through the membrane. The fact that it is possible to work at lower temperatures is an advantage over distillation. Moreover, mixture gaps and azeotropic points do not have to be taken into account.
RETENTION
In addition to its selectivity, the retention R of a membrane is a measure for the membrane’s separation efficiency. If mass fractions w are used for calculation, the following formula is derived for a component i. P stands for the permeate while F represents the feed.
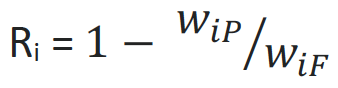
SELECTIVITY
The composition of feed and permeate defines the selectivity. For binary mixtures, the molar or mass fractions are often used. In contrast to the separation factor, the selectivity S of a membrane is therefore not obtained from measuring individual components, but mixtures. It can be determined using the following formula. i and j stand for the respective components in the gas mixture, where y and x represent the concentration of the components in permeate and feed respectively.
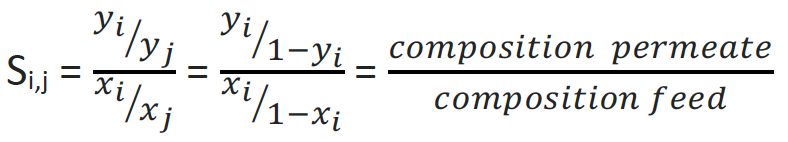
SEPARATION FACTOR
The separation factor of a membrane can be determined by measuring the permeation of individual gases on the membrane. The quotient of the determined permeabilities P of the gases is called the ideal separation factor α (see Formula 1), where i and j stand for the respective gases. The ideal separation factor of porous membranes is determined according to Formula 2. M1 and M2 represent the molar masses of the examined gases.
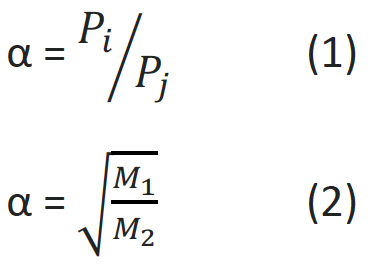
TRANSMEMBRANE PRESSURE
The transmembrane pressure (TMP) describes the difference in pressure of a component between the feed side and the permeate side of a membrane. For example, a TMP of 30 bar may indicate that a membrane is subjected to a gas pressure of 31 bar and to atmospheric pressure on the permeate side. However, a TMP of 30 bar can also prevail if gas is applied to the membrane at 60 bar and the permeate is removed from the permeate side with 30 bar sweep gas.


